By: George Mathew
Key takeaways
- Comprehensive Risk Management: Establishing a robust risk management framework is essential for mitigating risks associated with medical devices.
- Supplier Evaluation: Assessing supplier product quality and response times helps in selecting reliable vendors and ensuring timely procurement.
- Continuous Monitoring: Ongoing monitoring and review of the risk management process are necessary to adapt to changing risks and maintain compliance.
- Expert Assistance: Utilizing expert services like those from SpendEdge can enhance risk management capabilities through detailed supplier analysis and risk intelligence.
Risk management involves understanding and evaluating potential threats or opportunities and developing strategies to mitigate or capitalize on them. The goal of risk management is to minimize potential negative outcomes while maximizing potential positive outcomes. It helps you navigate through uncertain situations and make informed decisions to protect yourself or your organization.
What is medical device risk management?
Risk management in medical devices is crucial for the healthcare sector as patient safety is an utmost priority, in relation to medical devices the prime risk concerns are the quality of the equipment that is procured and the response time of the suppliers to its demand. Analyzing the potential risks associated with the procurement of medical equipment is necessary to start the reaction before time.
Effective medical device risk management
Supplier product quality assessment
A pharmaceutical facility’s first priority is product quality since subpar equipment runs the risk of endangering the life of a critical patient. Compromising with the best quality supplier can result in a loss of reputation, assessment of the supplier’s product quality and then selection of the best few is an effective risk management technique. The quality of the supplier’s product reflects the dedication of the vendor toward his work, a dedicated and professional vendor always maintains their quality of work. Assessing equipment quality guarantees the finest treatment of the patients with the help of A-one quality medical devices.
Supplier response time analysis
For managing the risk of delay in procurement, analyzing the response time of the potential suppliers can minimize the risk. The healthcare institution must evaluate the supplier’s prior performance records, obtain delivery schedules from them, and search for the ideal vendor who delivers at the scheduled time. Timely deliveries and responses can accelerate the process of procurement and most times quick treatment can result in less time for the recovery of a patient, early treatment can be achieved by on-time procurement of supplies.
Risk evaluation
Identifying the severity and occurrence probability of risks will make it easier for the facilities to quantify and evaluate them on time. Managing risk can be a time taking process if not evaluated in the required time. An effective risk management technique is evaluating the hazardous situation prior to its occurrence and taking necessary steps or measures to combat the risk. If the severity of the impact on the procurement process is analyzed by the healthcare sector, then immediate decisions can be made to minimize the effect.
Medical device risk management process
Step 1
To effectively manage the risks associated with medical devices, it is imperative to establish a comprehensive risk management process that aligns with regulatory standards such as those set forth by the FDA or ISO. This involves the creation of a risk management framework, which delineates the device development process and defines the roles and responsibilities of project stakeholders. Central to this framework is the development of a meticulously documented risk management plan.
Step 2
Conducting a thorough risk analysis for medical devices is paramount as it allows manufacturers to delineate the intended use of the product and focus on essential steps while identifying potential hazards. This process entails identifying foreseeable risks early in the development cycle, comprehensively detailing the causes, and projecting the potential consequences of the identified hazards.
Step 3
Quantifying and evaluating risks through the assessment of severity and occurrence is crucial in order to effectively prioritize risk mitigation efforts. Visualizing risks on a matrix empowers manufacturers to determine which hazards necessitate immediate attention based on their likelihood of occurrence and potential impact.
Step 4
Following the identification of risks, the subsequent step involves implementing robust risk mitigation strategies aimed at reducing the intensity of risks to an acceptable level. Mitigation efforts may encompass design changes, the integration of protective measures, or providing clear instructions and labeling in device manuals to address specific risks. Care must be taken when redesigning the product for risk control to avoid introducing new risks.
Step 5
Thorough documentation of the risk management plan and strategies is indispensable. This process extends throughout the entirety of the product development lifecycle and should encompass all actions, assessments, reports, and diagrams related to risk management. Moreover, it is crucial to maintain up-to-date records and to meticulously track the effectiveness of implemented control actions while monitoring resulting risks.
Challenges: Medical device risk management
Identifying potential risks
Recognizing all potential risks associated with a medical device can be challenging due to their complex, hidden nature and the fact that risks evolve over time.
Assessing the likelihood and severity of risks
Accurately gauging the likelihood and severity of risks is difficult, largely because of limited available data and the variability of influencing factors.
Implementing effective risk control measures
Effectively implementing risk control measures poses difficulties, as these measures can be costly, hard to execute, and not always guaranteed to be effective.
Monitoring and reviewing the risk management process
Continuous monitoring and reviewing of the risk management process is challenging due to its complexity and time-consuming nature, coupled with the need to keep up with changes in the product or environment.
Lack of resources
Medical device manufacturers often face a shortage of resources necessary for a thorough risk management process, including insufficient staff, time, or funding.
Lack of expertise
Manufacturers may lack the necessary expertise to implement a comprehensive risk management process, including knowledge of risk management principles and practices or experience in applying these to medical devices.
Regulatory requirements
Compliance with various regulatory requirements adds complexity and presents challenges to the risk management process for medical device manufacturers.
How SpendEdge can help with medical equipment risk assessment
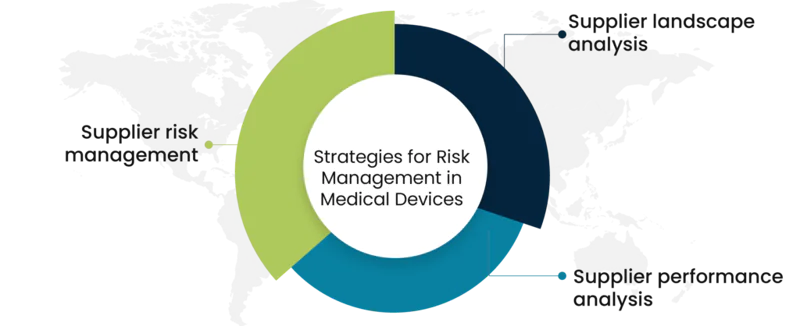
Supplier landscape analysis
We narrow down the potential supplier base by clearly analyzing the previous information of the vendors and streamlining the suppliers who can provide standard product quality. Gauge supplier capabilities based on key performance indicators, due diligence in the vendor onboarding process, and frequent verification of vendor data prevent you from supplier sub-standard product quality. You can identify the suppliers who can provide the best quality even in times of emergencies or volatile market conditions. Supplier landscape analysis conducted by us can provide you with the list of available suppliers who can confirm the consistently high quality of medical devices.
Supplier performance analysis
Our reports provide you with the past performance of the potential suppliers who stay ahead of the curve when it comes to on-time deliveries and responses, they ensure delivery of products on the promised time. You can sort suppliers based on operational and functional capabilities then rank them to identify the best-fit suppliers and get additional information about the most suitable suppliers for engagement. Gain valuable insights into prevalent market conditions which can ease supplier negotiations, supplier suitability can be evaluated using our information on strategic moves.
Supplier risk management
Our experts leverage statistics and techniques to make accurate predictions for multiple supplier risks and assign risk scores based on probability and severity. Supplier risk intelligence service can help you get alerts on vendor risks and will enable you to build up your onboard certified vendors. We help understand the mitigation measures adopted by suppliers to combat the risk, you can stay ahead of the competition by capitalizing on technology-enabled risk insights. Formulate decisive and sound measures to deal with procurement disruptions through our reports, this can ensure your growth with cost savings.
Success story: How SpendEdge helped a client with risk analysis in medical devices
Medical device risk assessment is significant for healthcare facilities because while procuring, the pharma industry may face difficulties like substandard quality or delay in deliveries. A pharmaceutical company must take the necessary steps to combat these medical devices’ supply chain risk.
Along the same lines, a pharmaceutical business contacted SpendEdge for assistance as they were dealing with a number of supplier concerns, such as late answers from the vendor and subpar product quality. They were interested in learning more about how the supplier market has changed.
To tackle these risks and help them in risk management we employed supplier risk intelligence to help the client get alerts on vendor risks. We leverage information and statistics to assist the company in analyzing the past performance of potential suppliers to collect knowledge about product quality and their response times. Our supplier market analysis provided the company with information on how the supplier market was changing.
The company was able to manage and minimize the risk in the procurement of medical devices through our expertise.
For any similar kind of issues, get in touch with us now, and make use of all our services to experience positive outcomes.
Conclusion
Effective risk management in the medical device sector is crucial for ensuring patient safety and maintaining the quality of healthcare services. By implementing a comprehensive risk management process that includes supplier product quality assessment, supplier response time analysis, and continuous monitoring, healthcare facilities can mitigate potential risks associated with medical device procurement. Despite the challenges such as limited resources, lack of expertise, and regulatory requirements, leveraging expert insights and data-driven approaches can significantly enhance risk management strategies. SpendEdge’s specialized services in supplier landscape analysis, performance analysis, and risk management provide invaluable support to healthcare organizations, enabling them to navigate procurement risks successfully and ensure optimal patient care.
Author’s details
George Mathew
Vice President, Sourcing and Procurement Intelligence
George is a procurement specialist at Infiniti Research and provides advisory services to clients across the pharmaceutical, CPG & FMCG, energy, and automotive sectors. He specializes in the procurement areas of industry benchmarking, cost modeling, rate card benchmarking, negotiation advisory, and supplier intelligence.




